True/False
Of the two molecules shown in the figure, one would expect that CO2 has a higher boiling point than NO2 because CO2 is linear and nonpolar. 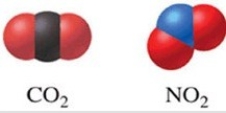
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q63: Which of the following has the highest
Q64: A solid substance has a moderate melting
Q65: Three phases of water are shown in
Q66: When liquid water is poured onto a
Q67: The forces that hold NH<sub>3</sub> together in
Q69: Identify the type of solid shown in
Q70: One would expect that water is more
Q71: Which of the following substances is most
Q72: A solid substance has a high melting
Q73: Which of the following statements regarding the