Multiple Choice
Upon electrification, hydrogen produces a line spectrum with the following lines in the visible region of the electromagnetic spectrum. If the light emitted corresponds to transitions from the third (n = 3) , fourth (n = 4) , fifth (n = 5) , or sixth (n = 6) energy level down to the second (n = 2) , which transition corresponds to the emission of light with lowest frequency? 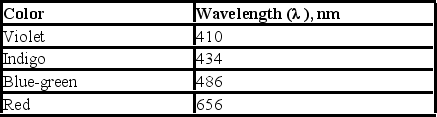
A) n = 2 → n = 3
B) n = 2 → n = 6
C) n = 3 → n = 2
D) n = 4 → n = 2
E) n = 6 → n = 2
Correct Answer:

Verified
Correct Answer:
Verified
Q83: Which element has two completely filled p
Q84: Which of the following statements regarding orbitals
Q85: Upon electrification, hydrogen produces a characteristic line
Q86: Which of the following is the correct
Q87: Which of the following is the correct
Q89: Some elements have electron configurations that deviate
Q90: Rank the following elements in order of
Q91: What is the abbreviated ground-state electron configuration
Q92: What is the abbreviated ground-state electron configuration
Q93: Which ion is expected to be the