Multiple Choice
Upon electrification, hydrogen produces a line spectrum with the following lines in the visible region of the electromagnetic spectrum. If the light emitted corresponds to transitions from the third (n = 3) , fourth (n = 4) , fifth (n = 5) , or sixth (n = 6) energy level down to the second (n = 2) , which transition corresponds to the emission of light with highest frequency? 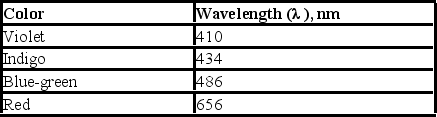
A) n = 2 → n = 3
B) n = 2 → n = 6
C) n = 3 → n = 2
D) n = 4 → n = 2
E) n = 6 → n = 2
Correct Answer:

Verified
Correct Answer:
Verified
Q61: Colored light from a heated ionic compound
Q62: Which element has the abbreviated ground-state electron
Q63: Rank the following elements in order of
Q64: Rank the following elements in order of
Q65: The green line observed in the line
Q67: Which of the following is the correct
Q68: The electron configuration 1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>6</sup>4s<sup>2</sup>3d<sup>10</sup>4p<sup>6</sup> applies to all
Q69: Which of the images represents an s
Q70: Rank the following elements in order of
Q71: The wavelength of the yellow light given