Multiple Choice
Upon electrification, hydrogen produces a line spectrum with the following lines in the visible region of the electromagnetic spectrum. If the light emitted corresponds to transitions from the third (n = 3) , fourth (n = 4) , fifth (n = 5) , or sixth (n = 6) energy level down to the second (n = 2) , which transition corresponds to the emission of light with highest energy? 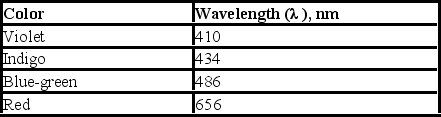
A) n = 2 → n = 3
B) n = 2 → n = 6
C) n = 3 → n = 2
D) n = 4 → n = 2
E) n = 6 → n = 3
Correct Answer:

Verified
Correct Answer:
Verified
Q37: Which element has the abbreviated ground-state electron
Q38: Rank the following types of electromagnetic radiation
Q39: Elements that have five electrons in the
Q40: If the energy of a photon of
Q41: Which of the following statements regarding spectra
Q43: Which of the following statements regarding orbitals
Q44: Some elements have electron configurations that deviate
Q45: When the elements lithium and fluorine react,
Q46: Write the element symbol for the element
Q47: The following orbital diagram corresponds to the