Multiple Choice
Which of the following is the best (simplest) balanced equation to represent the chemical reaction shown in the figure on any scale? 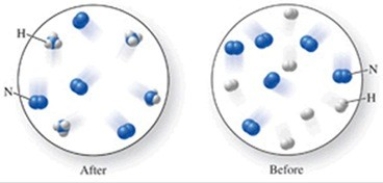
A) 12N + 12H → 12NH
B) 6N2 + 6H2 → 4NH3
C) 6N2 + 6H2 → 4NH3 + 4N2
D) 12N + 12H → 4NH3 + 8N
E) N2 + 3H2 → 2NH3
Correct Answer:

Verified
Correct Answer:
Verified
Q94: Given that 4NH<sub>3</sub>(g)+ 5O<sub>2</sub>(g)→ 4NO(g)+ 6H<sub>2</sub>O(g), if
Q95: Aluminum metal reacts with sulfuric acid according
Q96: Consider the following reaction: Cr<sub>2</sub>O<sub>3</sub>(s)+ 3CCl<sub>4</sub>(l)→ 2CrCl<sub>3</sub>(s)+
Q97: Which of the following processes is endothermic?<br>A)burning
Q98: Phosphorus trichloride can be made by the
Q100: An energy input of 227 kJ is
Q101: If the theoretical yield for a reaction
Q102: Iron metal reacts with chlorine gas according
Q103: Which of the following is an exothermic
Q104: The burning of a 2.50 g sample