Multiple Choice
When the mixture of molecules shown in the molecular-level image undergoes complete reaction, all of these molecules are converted to products. Which of the following reactions could this represent? 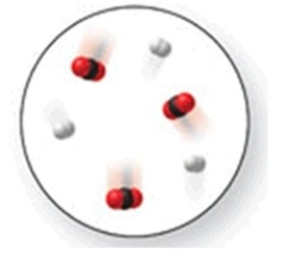
A) N2 + O2 → 2NO
B) N2 + 2Cl2 → N2Cl4
C) N2 + 2O2 → 2NO2
D) N2 + 3H2 → 2NH3
E) N2 + 3O2 → 2NO3
Correct Answer:

Verified
Correct Answer:
Verified
Q59: Small amounts of oxygen gas can be
Q60: How much heat energy would be needed
Q61: All of the following may change during
Q62: An aqueous solution containing 15.0 g of
Q63: Given that 4NH<sub>3</sub>(g)+ 5O<sub>2</sub>(g)→ 4NO(g)+ 6H<sub>2</sub>O(g), if
Q65: When sulfur dioxide is formed from its
Q66: When 3.0 mol CaCl<sub>2</sub> dissolves in water,
Q67: In a bomb calorimeter, the bomb itself,
Q68: Nitrogen and hydrogen react together to form
Q69: When fats or other foods are burned