Multiple Choice
The figure shows a molecular-level diagram of reactant molecules for the reaction 2H2(g) + O2(g) → 2H2O(l) 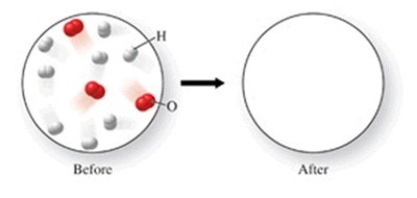 List the number and formulas of the molecules that should be present after the reaction takes place.
List the number and formulas of the molecules that should be present after the reaction takes place.
A) 2H2O + 6H2 + 2O2
B) 3H2O + 5H2 + O2
C) 4H2O + 4H2 + O2
D) 6H2O + 2H2 + O2
E) 6H2O + 2H2
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Consider the reaction between acetylene, C<sub>2</sub>H<sub>2</sub>, and
Q2: When sodium sulfate, Na<sub>2</sub>SO<sub>4</sub>, dissolves in water,
Q3: The coefficients of a balanced equation can
Q4: Phosphine, PH<sub>3</sub>, a reactive and poisonous compound,
Q6: Consider the reaction between sodium metal and
Q7: Consider the reaction between hydrogen and oxygen
Q8: When copper reacts with sulfur at high
Q9: The combustion of octane is described by
Q10: Aluminum reacts with oxygen according to the
Q11: Which of the following equations is balanced?<br>A)P<sub>4</sub>(s)+