Multiple Choice
The figure shows a molecular-level diagram of reactant molecules for the reaction: 2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(g) 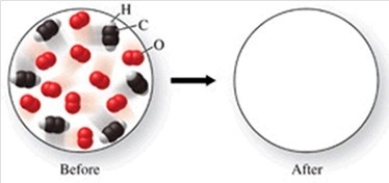 List the number and formulas of the molecules that should be present after the reaction takes place.
List the number and formulas of the molecules that should be present after the reaction takes place.
A) 4CO2 + 2H2O
B) 4CO2 + 2H2O + 2C2H2
C) 4CO2 + 2H2O + 2C2H2 + 5O2
D) 6CO2 + 3H2O + 3O2
E) 8CO2 + 4H2O + 2C2H2
Correct Answer:

Verified
Correct Answer:
Verified
Q79: When blue copper(II)sulfate pentahydrate is heated, it
Q80: When calculating the percent yield for a
Q81: Given that 4NH<sub>3</sub>(g)+ 5O<sub>2</sub>(g)→ 4NO(g)+ 6H<sub>2</sub>O(g), if
Q82: When a 0.525 g piece of zinc
Q83: Ammonia is usually made by the following
Q85: When mercury(II)oxide, a red crystalline solid, is
Q86: Phosphine, PH<sub>3</sub>, a reactive and poisonous compound,
Q87: A 2.50 g sample of pitted prunes
Q88: When mixed, solutions of silver nitrate, AgNO<sub>3</sub>,
Q89: The q value for the following reaction