Multiple Choice
Consider the reaction N2(g) + 2O2(g) → 2NO2(g) . The molecular image represents a mixture of N2(g) and O2(g) just before reaction occurs. What is the limiting reactant, and how much of the excess reactant remains after the reaction is complete? The image contains 3 N2 molecules and 9 O2 molecules. 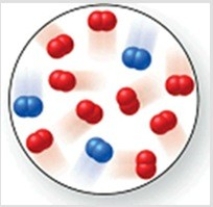
A) N2(g) , 6 O2(g)
B) O2(g) , 1 N2(g)
C) N2(g) , 3 O2(g)
D) O2(g) , 2 N2(g)
E) N2(g) , 7 O2(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q65: When sulfur dioxide is formed from its
Q66: When 3.0 mol CaCl<sub>2</sub> dissolves in water,
Q67: In a bomb calorimeter, the bomb itself,
Q68: Nitrogen and hydrogen react together to form
Q69: When fats or other foods are burned
Q71: When phosphorus reacts with chlorine, phosphorus trichloride
Q72: Consider the following reaction: 3NO<sub>2</sub>(g)+ H<sub>2</sub>O(l)→ 2HNO<sub>3</sub>(l)+
Q73: In photosynthesis, plants convert carbon dioxide and
Q74: Aluminum metal reacts with sulfuric acid according
Q75: When mixed, solutions of aluminum nitrate, Al(NO<sub>3</sub>)<sub>3</sub>,