Multiple Choice
In the figure shown, is a chemical reaction occurring? 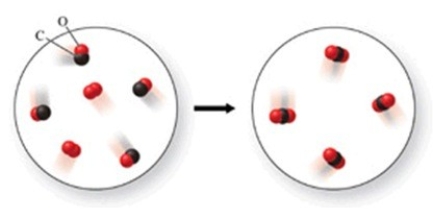
A) No, because there are the same number of atoms in both images.
B) Yes, because the atoms have rearranged to form a new substance.
C) No, because both the reactants and products are colorless gases.
D) No, because there are oxygen atoms and carbon atoms in both images.
E) Yes, because the reactants are gases, but the product is a solid.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: Which of the following changes represents a
Q16: After the following equation is properly balanced,
Q17: What is the class of the reaction
Q18: Examine the photograph. Note any evidence of
Q19: Balance the following skeletal equation: Pb(NO<sub>3</sub>)<sub>2</sub>(aq)+ KI(aq)→
Q21: When aqueous solutions of hydrochloric acid and
Q22: Consider the following chemical equations. Select the
Q23: The molecular-level diagram shows a combination reaction
Q24: Balance the following skeletal equation: CH<sub>4</sub>(g)→ C<sub>2</sub>H<sub>2</sub>(g)+
Q25: If solutions of potassium carbonate and calcium