Multiple Choice
The class of the reaction shown in the figure is 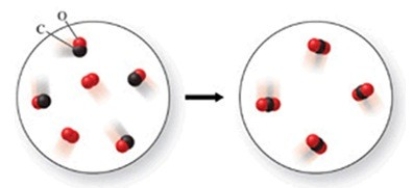
A) combustion reaction.
B) decomposition reaction.
C) double-displacement reaction.
D) single-displacement reaction.
E) combination reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q21: When aqueous solutions of hydrochloric acid and
Q22: Consider the following chemical equations. Select the
Q23: The molecular-level diagram shows a combination reaction
Q24: Balance the following skeletal equation: CH<sub>4</sub>(g)→ C<sub>2</sub>H<sub>2</sub>(g)+
Q25: If solutions of potassium carbonate and calcium
Q27: When two compounds react to form two
Q28: Predict which of the following reactions will
Q29: When one compound is converted into two
Q30: Consider the reaction Ca(OH)<sub>2</sub>(aq)+ 2HCl(aq)→ CaCl<sub>2</sub>(aq)+ 2H<sub>2</sub>O(l).
Q31: Consider the following chemical equations. Select the