Multiple Choice
The class of the reaction shown in the figure is 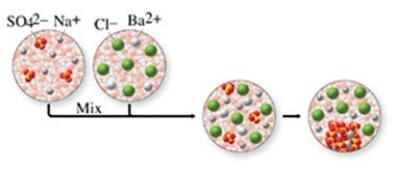
A) combination reaction.
B) combustion reaction.
C) single-displacement reaction.
D) double-displacement reaction.
E) decomposition reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q33: Examine the molecular-level diagram. Has a chemical
Q34: If solutions of potassium chromate and sodium
Q35: A solution of silver nitrate is mixed
Q36: Would an aqueous solution of (NH<sub>4</sub>)<sub>2</sub>CO<sub>3</sub> contain
Q37: When aqueous solutions of K<sub>2</sub>CO<sub>3</sub> and CaCl<sub>2</sub>
Q39: Which of the following is a balanced
Q40: What are the products of the combustion
Q41: The activity series is used to predict
Q42: Predict whether a reaction will occur when
Q43: Would an aqueous solution of MgBr<sub>2</sub> contain