Multiple Choice
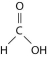
-The illustration above shows a representation of formic acid. A formic acid molecule
A) will form hydrogen bonds with water molecules.
B) has a tetrahedral configuration of hybrid electron orbitals for the carbon atom.
C) consists of largely nonpolar covalent bonds.
D) is held together by hydrogen bonds.
E) has a tetrahedral shape and will form hydrogen bonds with water molecules.
Correct Answer:

Verified
Correct Answer:
Verified
Q39: What is the maximum number of hydrogen
Q40: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" -Which drawing in
Q41: What results from an unequal sharing of
Q42: Trace elements are those required by an
Q43: Knowing just the atomic mass of an
Q45: The precise weight of a mole of
Q46: We can represent atoms by listing the
Q47: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" -Which drawing in
Q48: Why is each element unique and different
Q49: The atomic number of chlorine is 17.