Multiple Choice
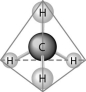
-What causes the shape of the molecule shown above?
A) the configuration of the 2 p orbitals in the carbon atom
B) the configuration of the 1 s orbital in the carbon atom
C) the configuration of the sp hybrid orbitals of the electrons shared between the carbon and hydrogen atoms
D) the packing of the carbon and hydrogen atoms in a crystal lattice
E) hydrogen bonding configurations between the carbon and hydrogen atoms
Correct Answer:

Verified
Correct Answer:
Verified
Q22: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" -Based on electron
Q23: Which of the following explains most specifically
Q24: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" -Which drawing in
Q25: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" -How many electrons
Q26: Which one of the atoms shown would
Q28: What is the atomic number of the
Q29: The atomic number of each atom is
Q30: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" -Which drawing in
Q31: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" -Refer to the
Q32: Which of the following correctly describes chemical