Multiple Choice
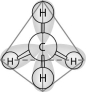
-In the methane molecule shown in the figure above, bonds have formed that include both the s orbital valence electrons of the hydrogen atoms and the p orbital valence electrons of the carbon. The electron orbitals in these bonds are said to be
A) double orbitals.
B) tetrahedral orbitals.
C) complex orbitals.
D) hybrid orbitals.
E) polar orbitals.
Correct Answer:

Verified
Correct Answer:
Verified
Q73: A group of molecular biologists is trying
Q74: The nucleus of a nitrogen atom contains
Q75: What coefficients must be placed in the
Q76: What is the maximum number of electrons
Q77: Carbon-12 is the most common isotope of
Q79: If an atom of sulfur (atomic number
Q80: Van der Waals interactions result when<br>A) hybrid
Q81: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" -In the figure
Q82: When two atoms are equally electronegative, they
Q83: Which of the following molecules contains the