Multiple Choice
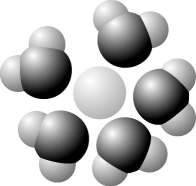
-Based on your knowledge of the polarity of water molecules, the solute molecule depicted here is most likely
A) positively charged.
B) negatively charged.
C) without charge.
D) hydrophobic.
E) nonpolar.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q30: Which of these molecules would be soluble
Q31: A solution contains 0.0000001(10⁻⁷)moles of hydroxyl ions
Q32: Carbon dioxide (CO₂)is readily soluble in water,
Q33: A strong acid like HCl<br>A) ionizes completely
Q34: The partial negative charge in a molecule
Q36: You have two beakers. One contains a
Q37: Equal volumes (5 mL)of vinegar from a
Q38: What is the pH of a 1
Q39: Why does evaporation of water from a
Q40: Assume that acid rain has lowered the