Multiple Choice
For the hydrolysis of ATP to ADP + 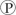 ᵢ, the free energy change is -7.3 kcal/mol under standard conditions (1 M concentration of both reactants and products) . In the cellular environment, however, the free energy change is about -13 kcal/mol. What can we conclude about the free energy change for the formation of ATP from ADP and
ᵢ, the free energy change is -7.3 kcal/mol under standard conditions (1 M concentration of both reactants and products) . In the cellular environment, however, the free energy change is about -13 kcal/mol. What can we conclude about the free energy change for the formation of ATP from ADP and 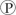 ᵢ under cellular conditions?
ᵢ under cellular conditions?
A) It is +7.3 kcal/mol.
B) It is less than +7.3 kcal/mol.
C) It is about +13 kcal/mol.
D) It is greater than +13 kcal/mol.
E) The information given is insufficient to deduce the free energy change.
Correct Answer:

Verified
Correct Answer:
Verified
Q55: A number of systems for pumping ions
Q56: Increasing the substrate concentration in an enzymatic
Q57: Which of the following statements regarding enzymes
Q58: Which of the following is true of
Q59: Which term most precisely describes the cellular
Q61: In order to attach a particular amino
Q62: The following questions are based on the
Q63: In experimental tests of enzyme evolution, where
Q64: During a laboratory experiment, you discover that
Q65: Chemical equilibrium is relatively rare in living