Multiple Choice
The following is an example of a first-order reaction involving the _____. 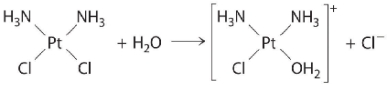
A) reaction of t-butyl bromide with water to give t-butanol
B) hydrolysis of the anticancer drug cisplatin
C) oxidation of ethanol to acetaldehyde in the liver
D) decomposition of N2O on a platinum surface
E) hydrolysis of aspirin
Correct Answer:

Verified
Correct Answer:
Verified
Q9: The rate of radioactive decay is dependent
Q10: A(n)_ rate law describes the reaction rate
Q11: The rate law for a reaction can
Q12: Irreversible inhibitors are therefore the equivalent of
Q13: Which heterogeneous catalyst is used in steam
Q15: Differential rate laws are generally used to
Q16: The reaction rate of a heterogeneous reaction
Q17: Which of the following best describes chemical
Q18: Reaction rates generally decrease with time as
Q19: A compound hydrolyzes in water with