Multiple Choice
Answer the questions based on the phase diagram given below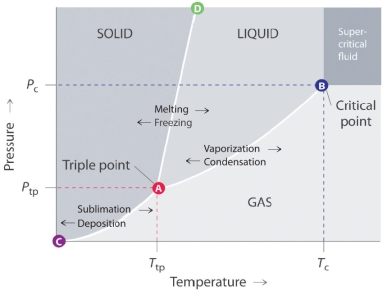
-The line that connects points A and B in the given phase diagram is:
A) is the vapor pressure curve of the solid phase.
B) shows the melting point variation of a solid with pressure.
C) is the vapor pressure curve of the liquid phase.
D) is the vapor pressure curve of the gas phase.
E) shows the boiling point variation of a liquid with pressure.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: The natural logarithm of 0.298 is _.<br>A)
Q5: The transition from a solid to a
Q6: Why do liquids adopt the shape, but
Q7: The enthalpy change that accompanies the conversion
Q8: When solid carbon dioxide is heated, it
Q10: In a phase diagram, the region that
Q11: Liquid crystals are isotropic.
Q12: The rate of diffusion in liquids is
Q13: The surface tension of a liquid is
Q14: An arrangement of molecules in which their