Multiple Choice
Answer the questions based on the phase diagram given below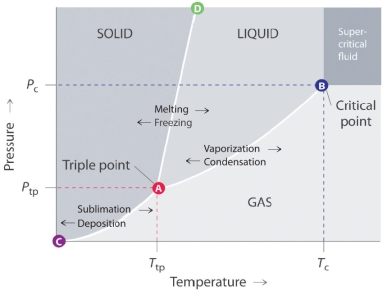
-Which of the following lines in the given phase diagram, represents the vapor pressure curve of the solid phase?
A) The line that connects points B and D.
B) The line that connects points C and B.
C) The line that connects points A and B.
D) The line that connects points A and C.
E) The line that connects points A and D.
Correct Answer:

Verified
Correct Answer:
Verified
Q85: _ are substances that are liquids at
Q86: London dispersion forces get stronger with increasing
Q87: A sphere has the smallest possible surface
Q88: A(n) _ is a graphic summary of
Q89: The _ refers to the pressure created
Q91: The single, dense fluid phase that exists
Q92: A substance that exhibits phases that have
Q93: A three fold increase in the distance
Q94: The arrangement of molecules in a liquid
Q95: The upper surface of a liquid in