Multiple Choice
Given: The Lewis structure of BeCl2. 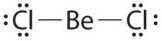 The structure shown above is an exception to the octet rule because the arrangement:
The structure shown above is an exception to the octet rule because the arrangement:
A) does not contain any double bonds.
B) gives each chlorine atom only eight electrons.
C) contains a beryllium atom.
D) contains only two chlorine atoms.
E) gives beryllium only four electrons.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: _ molecules are those which have less
Q7: The number of dots in the Lewis
Q8: Covalent bonding signifies that positively and negatively
Q9: Electron-deficient molecules are Lewis bases.
Q10: Triple bonds between like atoms are longer
Q12: Which of the following is used
Q13: Energy of the electrostatic attraction (E), a
Q14: The _ is a balance between the
Q15: Explain the three features of chemical bonding.
Q16: Which of the following is true of