Multiple Choice
In the equation given below, cyclopentanol (molar mass = 86.1 g/mol) reacts with pentanoic acid (molar mass = 102 g/mol) to produce cyclopentanoate(molar mass = 170.2 g/mol) and water. The volume of 0.297 M pentanoic acid needed for the complete reaction of 40.0 g cyclopentanol is _____ mL. 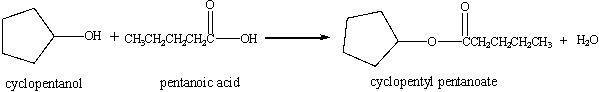
A) 0.86 × 103
B) 5.78 × 103
C) 7.58 × 103
D) 1.56 × 103
E) 0.76 × 103
Correct Answer:

Verified
Correct Answer:
Verified
Q75: Given: CH<sub>3</sub>CH<sub>2</sub>COOH + H<sub>2</sub>O <span
Q76: Given: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8341/.jpg" alt="Given: If
Q77: Which of the following is a nonelectrolyte?<br>A)
Q78: The ions that do not participate in
Q79: Which of the following is an example
Q81: _ is the crystalline solid formed when
Q82: In chemistry, the _ of a solution
Q83: Differentiate between an overall chemical equation and
Q84: The precipitate formed when an aqueous solution
Q85: _ is a monoprotic acid.<br>A) Sulfuric acid<br>B)