Multiple Choice
What value of Z (atomic number) and A (mass number) result in the following alpha decay? 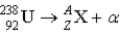
A) Z = 92; A = 238
B) Z = 91; A = 238
C) Z = 90; A = 234
D) Z = 93; A = 238
E) Z = 88; A = 236
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q35: Calculate the binding energy per nucleon (MeV/nucleon)
Q36: How many radioactive atoms are present in
Q37: When a neutron decays, a proton and
Q38: It is often possible to use atomic
Q39: The theory of nuclear astrophysics is that
Q41: Radiant energy reaching the Earth from the
Q42: The isotope, tritium, has a half-life of
Q43: A self-sustained chain reaction occurs when the
Q44: How many grams of deuterium (atomic mass
Q45: The half-life of <sup>131</sup>I is 8 days.