Multiple Choice
A glass container holds equal numbers of atoms of phosphorus 30 with a half-life of 2.5 minutes and of nitrogen 13 with a half-life of 10 minutes. After 20 minutes the ratio of the number of nitrogen atoms remaining to the number of phosphorus atoms remaining is
A) 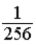 .
.
B) 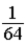 .
.
C) 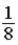 .
.
D) 64.
E) 256.
Correct Answer:

Verified
Correct Answer:
Verified
Q21: The reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8245/.jpg" alt="The reaction
Q22: When a beam of nuclear radiation of
Q23: The nuclear probability of interacting with neutrons
Q24: A neutron is characterized by the term
Q25: 44 g of petrified wood was found
Q27: Background radiation from cosmic rays and radioactive
Q28: Two nuclei which share the same atomic
Q29: One roentgen is defined as<br>A) the amount
Q30: A principal mechanism for energy loss during
Q31: How much energy (in MeV) is released