Multiple Choice
In the Lennard-Jones model of the hydrogen molecule, the potential is given by 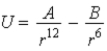 . In this model, the minimum internuclear separation, r0, is
. In this model, the minimum internuclear separation, r0, is
A) 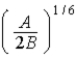 .
.
B) 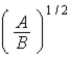 .
.
C) 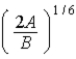 .
.
D) 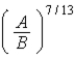 .
.
E) 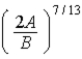 .
.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: In comparing vibrational and rotational levels in
Q27: An LED emits light of wavelength 600
Q28: The rotation spectrum of the HCl molecule
Q29: How many degrees of freedom does a
Q30: The frequency of a microwave absorbed by
Q32: To find the number of electrons per
Q33: The fundamental frequency of HF is 8.72
Q34: The smallest object one can distinguish using
Q35: Because HF, hydrogen fluoride, is a covalent
Q36: The Fermi energy corresponds to<br>A) the maximum