Multiple Choice
When a molecule jumps from a rotational energy level characterized by the rotational quantum number J to one characterized by J + 1, the change in energy, EJ + 1 − EJ, is
A) 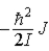 .
.
B) 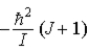 .
.
C) 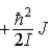 .
.
D) 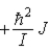 .
.
E) 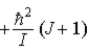 .
.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: When a molecule jumps from a rotational
Q19: The Fermi temperature is<br>A) a characteristic temperature
Q20: A diatomic molecule consists of two point
Q21: The energy of a molecule can normally
Q22: The dissociation energy of the hydrogen molecule
Q24: An oxygen molecule has a moment of
Q25: The Fermi energy of a metal at
Q26: In comparing vibrational and rotational levels in
Q27: An LED emits light of wavelength 600
Q28: The rotation spectrum of the HCl molecule