Multiple Choice
The diagram below shows the distance between the nuclei, pA and pB, and the electrons, e1 and e2, in a hydrogen molecule. We would expect the electrostatic potential energy of this molecule to be 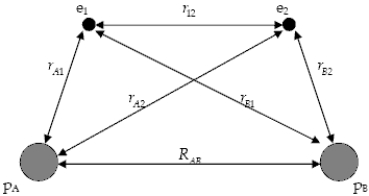
A) 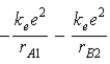 .
.
B) 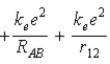 .
.
C) 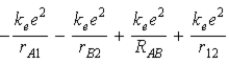 .
.
D) 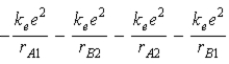 .
.
E) 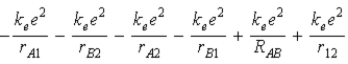 .
.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q36: The Fermi energy corresponds to<br>A) the maximum
Q37: When a voltage ΔV is applied to
Q38: Solid argon has a density of 1650
Q39: The Fermi temperature of copper is 80
Q40: Ellis and Randy are looking at a
Q42: The energy released when an atom takes
Q43: The fundamental frequency of CO is 6.42
Q44: Which of the following refer to the
Q45: The difference between donor and acceptor atoms
Q46: The wave functions of some molecules are