Multiple Choice
In terms of a0, where a0 = 0.0529 nm, the radii of the allowed orbits in the Bohr model of the hydrogen atom are given by rn =
A) 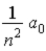 .
.
B) 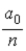 .
.
C) 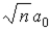 .
.
D) na0.
E) n2a0.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Of the following states, 5s, 3p, 4f,
Q9: In 1921, Stern and Gerlach performed an
Q10: What wavelength (in μm) is associated with
Q11: In an atom that has an electron
Q12: An electron is moving at a speed
Q14: Zeke says that the magnitude of the
Q15: The Pauli Exclusion Principle states<br>A) no two
Q16: What is the difference in frequency for
Q17: What angle does the orbital angular momentum
Q18: A hydrogen atom in the 4f state