Multiple Choice
In an allowed electron transition in a hydrogen atom,
A) Δ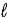 = 0;
= 0;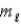 = 0, ±1.
= 0, ±1.
B) Δ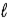 = 0, ±1;
= 0, ±1;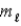 = ±1.
= ±1.
C) Δ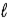 = 0, ±1;
= 0, ±1;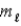 = 0, ±1.
= 0, ±1.
D) Δ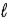 = ±1;
= ±1;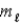 = 0, ±1.
= 0, ±1.
E) Δ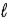 = ±1;
= ±1;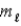 = ±1.
= ±1.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q44: The ground state configuration of chlorine (Z
Q45: An energy of 13.6 eV is needed
Q46: Which of the following, in which n
Q47: The magnitude of the spin angular momentum
Q48: All quantum states forming a shell have
Q50: What value of wavelength is associated with
Q51: The energy needed to change a He<sup>+</sup>
Q52: The allowed values of n for the
Q53: The energy needed to remove an electron
Q54: Rubidium (Z = 37) and potassium (Z