Multiple Choice
If n moles of an ideal gas are compressed isothermally from an initial volume V1 to a final volume V2, the change in entropy is
A) nR ln (V2/V1)
B) nRT ln (V2/V1)
C) nkB ln (V2/V1)
D) 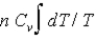
E) n Cv/T
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: The change in entropy when 1 kg
Q34: In an engine operating in the Otto
Q35: Which of the following is an almost
Q36: Since L<sub>ice</sub> = 333 J/g, the change
Q37: A vessel containing 5.0 kg of water
Q39: A heat pump has a coefficient of
Q40: A violation of the second law of
Q41: An engine is designed to obtain energy
Q42: By operating a reversible heat engine with
Q43: The change in entropy of 1.00 kg