Multiple Choice
Selena states that 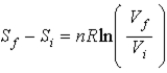 proves that entropy has a definite value at the beginning and end of an adiabatic free expansion. Ron says
proves that entropy has a definite value at the beginning and end of an adiabatic free expansion. Ron says 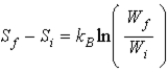 , where W is the number of microstates of a given macrostate. Which one, if either, is correct?
, where W is the number of microstates of a given macrostate. Which one, if either, is correct?
A) Only Selena, because entropy can depend only on macroscopic variables.
B) Only Ron, because entropy can depend only on microscopic variables.
C) Only Selena, because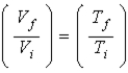 in an adiabatic free expansion.
in an adiabatic free expansion.
D) Neither, because we cannot calculate changes in entropy in an adiabatic free expansion.
E) Both, because entropy, which is macroscopic is a function of microscopic disorder.
Correct Answer:

Verified
Correct Answer:
Verified
Q26: For the same temperature increase in a
Q27: When water of mass m and specific
Q28: Ten kilograms of water at 0°C is
Q29: Exactly 500 grams of ice are melted
Q30: Suppose there are 3 molecules in a
Q32: An ideal gas is allowed to expand
Q33: The change in entropy when 1 kg
Q34: In an engine operating in the Otto
Q35: Which of the following is an almost
Q36: Since L<sub>ice</sub> = 333 J/g, the change