Multiple Choice
The average molecular translational kinetic energy of a molecule in an ideal gas is
A) 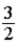 kBT.
kBT.
B) 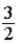 RT.
RT.
C) 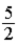 kBT.
kBT.
D) 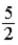 RT.
RT.
E) 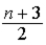 kBT, where n = number of internal degrees of freedom.
kBT, where n = number of internal degrees of freedom.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: The air in an automobile engine at
Q19: Suppose a box contains about 5.0 ×
Q20: Two tanks of gas, one of hydrogen,
Q21: If the rms speed of helium atoms
Q22: The average translational speed of a nitrogen
Q24: During an adiabatic compression, a volume of
Q25: A container having a volume of 1.0
Q26: The internal energy of n moles of
Q27: Two tanks of gas, one of hydrogen,
Q28: The molar specific heat at constant pressure