Multiple Choice
The molar specific heat at constant volume at 0°C of an ideal diatomic gas is
A) 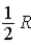 .
.
B) R.
C) 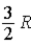 .
.
D) 2R.
E) 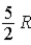 .
.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: During the volcanic eruption of Mt. Pelee
Q6: Nitrogen gas is heated by a pulsed
Q7: Air expands adiabatically (no heat in, no
Q8: When we say that the speed of
Q9: Find the specific heat (in cal/mole K)
Q11: One mole of hydrogen, one mole of
Q12: The molar specific heat at constant volume
Q13: A 50-gram sample of dry ice (solid
Q14: The root mean square speed of a
Q15: Assume 3.0 moles of a diatomic gas