Multiple Choice
Radioactivity: The stability of 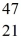 Sc with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
Sc with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 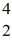 He: 4.002603 u
He: 4.002603 u 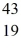 K: 42.960717 u
K: 42.960717 u 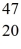 Ca: 46.954543 u
Ca: 46.954543 u 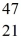 Sc 46.952409 u
Sc 46.952409 u 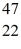 Ti: 46.951764 u The
Ti: 46.951764 u The 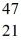 Sc nuclide is
Sc nuclide is
A) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+ decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.
Correct Answer:

Verified
Correct Answer:
Verified
Q17: Biomedicine: A 70-kg laboratory technician absorbs 2.9
Q18: Radioactive dating: An ancient rock is found
Q19: Properties of the nucleus: A certain nucleus
Q20: Radioactive dating: The radioactivity due to carbon-14
Q21: Radioactivity: If a nucleus decays by gamma
Q23: Nuclear fission: How much energy is released
Q24: Radioactive decay: A radioactive nuclide of atomic
Q25: Biomedicine: A 57-kg researcher absorbs 6.3 ×
Q26: Radioactivity: If a nucleus decays by alpha
Q27: Nuclear reactions: In the nuclear reaction <img