Multiple Choice
Radioactivity: The stability of 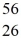 Fe with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
Fe with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 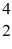 He: 4.002603 u
He: 4.002603 u 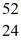 Cr: 51.944768 u
Cr: 51.944768 u 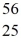 Mn: 55.938907 u
Mn: 55.938907 u 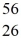 Fe: 55.934939 u
Fe: 55.934939 u 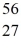 Co: 55.939841 u The
Co: 55.939841 u The 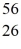 Fe nuclide is
Fe nuclide is
A) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.
Correct Answer:

Verified
Correct Answer:
Verified
Q40: Nuclear binding energy: Heavier stable nuclei tend
Q41: Nuclear fission: In the fission reaction <img
Q42: Nuclear reactions: A proton strikes an <img
Q43: Nuclear binding energy: How much energy is
Q44: Nuclear fission: Which of the following descriptions
Q46: Radioactivity: A radioactive atom has 98 protons
Q47: Nuclear reactions: For the missing product X
Q48: Radioactivity: If a nucleus decays by β<sup>-
Q48: Radioactivity: If a nucleus decays by β<sup>-
Q49: Radioactive decay: How many days are required