Multiple Choice
Reaction energy: Find the reaction energy (Q value) of the reaction 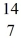 N +
N + 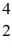 He →
He → 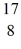 O +
O + 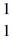 H, given the following masses:
H, given the following masses: 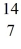 N: 14.003074 u
N: 14.003074 u 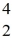 He: 4.002603 u
He: 4.002603 u 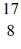 O : 16.999131 u
O : 16.999131 u 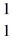 H: 1.007825 u (1 u = 931.5 MeV/c2)
H: 1.007825 u (1 u = 931.5 MeV/c2)
A) -1.191 MeV
B) -2.030 MeV
C) -3.241 MeV
D) -6.724 MeV
E) -9.055 MeV
Correct Answer:

Verified
Correct Answer:
Verified
Q32: Reaction energy: The detonation of a certain
Q33: Nuclear fission: An excited <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8274/.jpg" alt="Nuclear
Q34: Reaction energy: The reaction energy (Q value)
Q35: Nuclear fusion: In massive stars, three helium
Q36: Radioactive decay: The unstable isotope <sup>234</sup>Th decays
Q38: Radioactive dating: Living matter has 1.3 ×
Q39: Nuclear binding energy: What is the binding
Q40: Nuclear binding energy: Heavier stable nuclei tend
Q41: Nuclear fission: In the fission reaction <img
Q42: Nuclear reactions: A proton strikes an <img