Short Answer
Nuclear fusion: Consider the fusion reaction 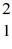 H +
H + 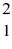 H +
H + 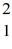 H →
H → 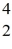 He +
He + 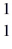 H +
H + 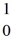 n
n
The atomic masses are: 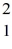 H: 2.01410 u
H: 2.01410 u 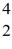 He: 4.00260 u
He: 4.00260 u 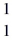 H, 1.00783 u
H, 1.00783 u 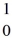 n: 1.008665 u
n: 1.008665 u
What mass of deuterium fuel, 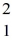 H, is used up in producing 8.2 × 1013 J of energy by this reaction? (1u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
H, is used up in producing 8.2 × 1013 J of energy by this reaction? (1u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
Correct Answer:

Verified
Correct Answer:
Verified
Q55: Radioactive dating: Carbon-14 has a half-life of
Q56: Radioactive decay: The decay rate of an
Q57: Nuclear fusion: A fusion reaction releases energy
Q58: Radioactive decay: The half-life of cobalt-60 is
Q59: Radioactive decay: A radioactive sample has a
Q61: Radioactivity: The stability of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8274/.jpg" alt="Radioactivity:
Q62: Radioactive decay: A hospital patient has been
Q63: Biomedicine: The maximum permissible workday dose for
Q64: Nuclear fusion: The reaction <sup>2</sup>H + <sup>2</sup>H
Q65: Radioactive decay: Rutherfordium-261 has a half-life of