Multiple Choice
Quantum numbers: If an electron has spin quantum number ms = - 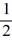 , what is the possible value for the orbital quantum number l of the electron?
, what is the possible value for the orbital quantum number l of the electron?
A) 0
B) 1
C) 2
D) 11
E) All of the above numbers are possible.
Correct Answer:

Verified
Correct Answer:
Verified
Q29: Multielectron atoms: The correct ground state electron
Q30: Multielectron atoms: An atom has completely filled
Q31: Multielectron atoms: What is the wavelength of
Q32: Multielectron atoms: The only INVALID electron state
Q33: Zeeman effect: The energy of an electron
Q35: Lasers: A collection of atoms has 20%
Q36: Lasers: Which of the following are characteristics
Q37: Hydrogen atom: The binding energy of the
Q38: Multielectron atoms: Which of the following electron
Q39: Multielectron atoms: What is the correct electronic