Multiple Choice
Quantum numbers: In the ground state, the quantum numbers (n, l, ml, ms) for hydrogen are, respectively
A) 1, 1, 1, 1.
B) 1, 0, 0, 0.
C) 1, 0, 0, ± 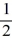 .
.
D) 1, 1, 1, ± 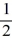 .
.
E) 1, 1, 0, ± 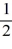
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: Hydrogen atom: What is the minimum speed
Q22: Hydrogen atom: What is the energy of
Q23: Quantum numbers: For each value of the
Q24: Multielectron atoms: An electron initially in a
Q25: Angular momentum: The magnitude of the orbital
Q27: Zeeman effect: An alkali metal atom is
Q28: Quantum numbers: An electron in a hydrogen
Q29: Multielectron atoms: The correct ground state electron
Q30: Multielectron atoms: An atom has completely filled
Q31: Multielectron atoms: What is the wavelength of