Multiple Choice
Angular momentum: What is the greatest magnitude of the orbital angular momentum L for an electron in a state with principal quantum number 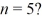
A) 4.47 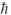
B) 4.90 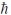
C) 5 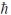
D) 5.48 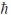
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Hydrogen atom: The normalized wave function for
Q3: Zeeman effect: Electrons in the presence of
Q4: Multielectron atoms: A neutral atom has an
Q5: Angular momentum: The magnitude of the orbital
Q6: Multielectron atoms: Consider the n = 9
Q7: Lasers: In a ruby laser, an electron
Q8: Zeeman effect: An alkali metal atom is
Q9: Zeeman effect: An atom in a state
Q10: Multielectron atoms: An atom has completely filled
Q11: Lasers: How many photons per second emerge