Multiple Choice
Bohr atom: A hydrogen atom is in its n = 2 excited state when its electron absorbs a photon of energy 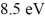 . What is the energy of the resulting free electron? The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
. What is the energy of the resulting free electron? The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 5.1 eV
B) 6.6 eV
C) 6.9 eV
D) 7.7 eV
Correct Answer:

Verified
Correct Answer:
Verified
Q51: Matter waves: A nonrelativistic electron has a
Q52: Photoelectric effect: When a metal surface is
Q53: Blackbody radiation: An electric current through a
Q54: Uncertainty principle using ΔxΔp ≥ ħ: A
Q55: Uncertainty principle using ΔpΔx ≥ h/2: A
Q57: Compton scattering: X-rays of energy 2.9 ×
Q58: Blackbody radiation: What is the wavelength of
Q59: Photoelectric effect: Light of wavelength 400 nm
Q60: Uncertainty principle using ΔEΔt ≥ ħ/2: A
Q61: Matter waves: What is the energy of