Multiple Choice
Bohr atom: A hydrogen atom makes a downward transition from the 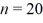 state to the n = 5 state. Find the wavelength of the emitted photon. The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J, c = 3.00 × 108 m/s)
state to the n = 5 state. Find the wavelength of the emitted photon. The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J, c = 3.00 × 108 m/s)
A) 2.43 μm
B) 1.46 μm
C) 1.94 μm
D) 2.92 μm
Correct Answer:

Verified
Correct Answer:
Verified
Q45: Uncertainty principle using ΔpΔx ≥ h/2: A
Q46: Photon energy: Gamma rays are photons with
Q47: Bohr atom: A hydrogen atom is excited
Q48: Uncertainty principle using ΔpΔx ≥ h/2: A
Q49: Uncertainty principle using ΔpΔx ≥ ħ/2: A
Q51: Matter waves: A nonrelativistic electron has a
Q52: Photoelectric effect: When a metal surface is
Q53: Blackbody radiation: An electric current through a
Q54: Uncertainty principle using ΔxΔp ≥ ħ: A
Q55: Uncertainty principle using ΔpΔx ≥ h/2: A