Multiple Choice
Quantity of heat: A 648-g empty iron kettle is put on a stove. How much heat. in joules. must it absorb to raise its temperature from 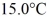 to
to 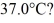 (The specific heat for iron is 113 cal/kg ∙ °C, 1 cal = 4.190 J)
(The specific heat for iron is 113 cal/kg ∙ °C, 1 cal = 4.190 J)
A) 6740 J
B) 11,300 J
C) 1610 J
D) 16,100 J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q82: Phase changes: A substance has a melting
Q83: Thermal expansion: You want to insert an
Q84: Molecular speeds: The average molecular kinetic energy
Q85: Ideal gas law: How many moles of
Q86: Thermal expansion: A brass rod is 40.1
Q88: Molecular speeds: A cubic box with sides
Q89: Mean free path: What is the mean
Q90: Radiation: A spherical object 25.0 cm in
Q91: Molecular speeds: The root-mean-square speed (thermal speed)
Q92: Calorimetry: Two experimental runs are performed to