Multiple Choice
Calorimetry: A 200-g metal container, insulated on the outside, holds 100 g of water in thermal equilibrium at 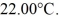 A 21-g ice cube, at the melting point, is dropped into the water, and when thermal equilibrium is reached the temperature is 15.00°C. Assume there is no heat exchange with the surroundings. For water, the specific heat is 4190 J/kg · K and the heat of fusion is 3.34 × 105 J/kg. The specific heat for the metal is closest to
A 21-g ice cube, at the melting point, is dropped into the water, and when thermal equilibrium is reached the temperature is 15.00°C. Assume there is no heat exchange with the surroundings. For water, the specific heat is 4190 J/kg · K and the heat of fusion is 3.34 × 105 J/kg. The specific heat for the metal is closest to
A) 3850 J/kg ∙ K.
B) 2730 J/kg ∙ K.
C) 4450 J/kg ∙ K.
D) 4950 J/kg ∙ K.
E) 5450 J/kg ∙ K.
Correct Answer:

Verified
Correct Answer:
Verified
Q53: Radiation: A blacksmith is flattening a steel
Q54: Molecular speeds: An oxygen molecule falls in
Q55: Ideal gas law: A 3.2-L volume of
Q56: Radiation: A cube at 100.0°C radiates heat
Q57: Ideal gas law: 3.00 moles of an
Q59: Quantity of heat: It is a well-known
Q60: Thermal expansion: A certain metal has a
Q61: Conduction of heat: Two metal rods, one
Q62: Conduction of heat: A heat conducting rod,
Q63: Ideal gas law: A weather balloon contains