Short Answer
Molecular speeds: What is the total translational kinetic energy in a test chamber filled with nitrogen (N2) at 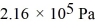 and
and 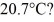 The dimensions of the chamber are
The dimensions of the chamber are 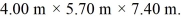 The ATOMIC weight of nitrogen is 28.0 g/mol, Avogadro's number is 6.022 × 1023 molecules/mol and the Boltzmann constant is 1.38 × 10-23 J/K.
The ATOMIC weight of nitrogen is 28.0 g/mol, Avogadro's number is 6.022 × 1023 molecules/mol and the Boltzmann constant is 1.38 × 10-23 J/K.
Correct Answer:

Verified
Correct Answer:
Verified
Q75: Conduction of heat: What is the steady
Q76: Thermal expansion: A glass flask has a
Q77: Molecular speeds: At what temperature would the
Q78: Molecular speeds: The root-mean-square speed (thermal speed)
Q79: Calorimetry: Two experimental runs are performed to
Q81: Mean free path: What is the mean
Q82: Phase changes: A substance has a melting
Q83: Thermal expansion: You want to insert an
Q84: Molecular speeds: The average molecular kinetic energy
Q85: Ideal gas law: How many moles of