Multiple Choice
Heat engines: A heat engine takes 2.0 moles of an ideal gas through the reversible cycle abca, on the pV diagram shown in the figure. The path bc is an isothermal process. The temperature at c is 820 K, and the volumes at a and c are 0.010 m3 and 0.16 m3, respectively. The molar heat capacity at constant volume, of the gas, is 37 J/mol∙K, and the ideal gas constant is R = 8.314 J/(mol∙K) . The thermal efficiency of the engine is closest to 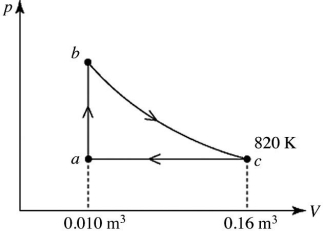
A) 0.26.
B) 0.026.
C) 0.33.
D) 0.40.
E) 0.53.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Entropy: What is the change in entropy
Q10: Heat pumps: An air conditioner with a
Q11: Heat engines: A real (non-Carnot) heat engine,
Q12: Refrigerators: A refrigerator removes heat from the
Q13: Entropy: At atmospheric pressure, 45 moles of
Q15: Carnot devices: A Carnot refrigerator has a
Q16: Heat engines: Is it possible to transfer
Q17: Entropy: A 2.00-kg block of ice at
Q18: Carnot engine: A Carnot cycle engine operates
Q19: Carnot engine: A Carnot engine operates between