Multiple Choice
Entropy: A 810-g quantity of ethanol, in the liquid state at its melting point of 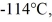 is frozen at atmospheric pressure. The heat of fusion of ethanol is 1.04 × 105 J/kg, the molecular mass is 46.1 g/mol, and the ideal gas constant is R = 8.314 J/(mol∙K) . The change in the entropy of the ethanol as it freezes is closest to
is frozen at atmospheric pressure. The heat of fusion of ethanol is 1.04 × 105 J/kg, the molecular mass is 46.1 g/mol, and the ideal gas constant is R = 8.314 J/(mol∙K) . The change in the entropy of the ethanol as it freezes is closest to
A) -540 J/K.
B) -490 J/K.
C) -600 J/K.
D) 490 J/K.
E) 540 J/K.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Entropy: A 2.0-kg block of aluminum at
Q3: Heat engines: A heat engine with an
Q4: Entropy: The entropy of an isolated system
Q5: Entropy: A system consists of two very
Q6: Refrigerators: During each cycle of operation, a
Q8: Refrigerators: A refrigerator has a coefficient of
Q9: Entropy: What is the change in entropy
Q10: Heat pumps: An air conditioner with a
Q11: Heat engines: A real (non-Carnot) heat engine,
Q12: Refrigerators: A refrigerator removes heat from the