Multiple Choice
Molar heat capacities: The temperature of an ideal gas in a sealed 0.40 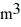 container is reduced from 400 K to
container is reduced from 400 K to 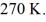 The final pressure of the gas is
The final pressure of the gas is 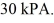 The molar heat capacity at constant volume of the gas is 28.0 J/mol · K. The work done by the gas is closest to
The molar heat capacity at constant volume of the gas is 28.0 J/mol · K. The work done by the gas is closest to
A) 0.00 kJ.
B) -19 kJ.
C) -25 kJ.
D) 19 kJ.
E) 25 kJ.
Correct Answer:

Verified
Correct Answer:
Verified
Q11: First law of thermodynamics: When a fixed
Q12: Molar heat capacities: The temperature of an
Q13: First law of thermodynamics: An ideal gas
Q14: Types of thermodynamic processes: When a gas
Q15: First law of thermodynamics: During an isothermal
Q17: Work: How much work is done by
Q18: Type of thermodynamic processes: The figure shows
Q19: Molar heat capacities: An ideal monatomic gas
Q20: Molar heat capacities: An expansion process on
Q21: Types of thermodynamic processes: The process shown