Multiple Choice
Molar heat capacities: A compression, at a constant pressure of 190 kPa, is performed on 5.0 moles of an ideal monatomic gas. The compression reduces the volume of the gas from 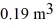 to
to 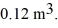 The ideal gas constant is R = 8.314 J/mol ∙ K. The work done by the gas is closest to
The ideal gas constant is R = 8.314 J/mol ∙ K. The work done by the gas is closest to
A) -13 kJ.
B) 13 kJ.
C) -33 kJ.
D) 33 kJ.
E) 0.00 kJ.
Correct Answer:

Verified
Correct Answer:
Verified
Q32: First law of thermodynamics: When an ideal
Q33: Type of thermodynamic processes: The figure shows
Q34: Molar heat capacities: A certain amount of
Q35: Types of thermodynamic processes: The process shown
Q36: Type of thermodynamic processes: The figure shows
Q38: First law of thermodynamics: During an adiabatic
Q39: Molar heat capacities: An ideal gas with
Q40: First law of thermodynamics: A container with
Q41: First law of thermodynamics: An ideal gas
Q42: Molar heat capacities: 3.0 moles of an