Multiple Choice
Molar heat capacities: A monatomic ideal gas undergoes an isothermal expansion at 300 K, as the volume increased from 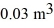 to
to 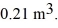 The final pressure of the gas is
The final pressure of the gas is 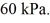 The ideal gas constant is R = 8.314 J/mol ∙ K. The change in the internal (thermal) energy of the gas is closest to
The ideal gas constant is R = 8.314 J/mol ∙ K. The change in the internal (thermal) energy of the gas is closest to
A) 0.00 kJ.
B) 12 kJ.
C) 25 kJ.
D) -12 kJ.
E) -25 kJ.
Correct Answer:

Verified
Correct Answer:
Verified
Q24: First law of thermodynamics: The gas in
Q25: First law of thermodynamics: A cylinder contains
Q26: Type of thermodynamic processes: The figure (not
Q27: First law of thermodynamics: When a fixed
Q28: Molar heat capacities: A quantity of ideal
Q30: First law of thermodynamics: When a fixed
Q31: First law of thermodynamics: A fixed amount
Q32: First law of thermodynamics: When an ideal
Q33: Type of thermodynamic processes: The figure shows
Q34: Molar heat capacities: A certain amount of